Abstract
Introduction:
Osteolytic bone disease is the most common clinical feature of multiple myeloma (MM), affecting approximately 80% of patients (pts) at diagnosis. Non-invasive biomarkers of bone resorption and formation are indicative of bone dynamics and have been used to assess bone metabolism during anti-myeloma treatment. Daratumumab (dara), an IgG1 κ human monoclonal antibody targeting CD38, is approved as treatment of patients with relapsed and/or refractory MM (RRMM). Moreover, dara inhibits in vitro osteoclastogenesis and bone resorption. The present study investigated the impact of dara monotherapy on bone remodeling in pts with RRMM who have had ≥2 prior lines of therapy.
Methods:
REBUILD was a prospective, non-comparative, open-label, phase 2 study, conducted in six centers in Greece. Eligible pts were adults with documented RRMM, who have had ≥2 prior lines of therapy (including lenalidomide and a PI), evidence of progressive disease by International Myeloma Working Group criteria, a Karnofsky Performance Status score ≥70, and creatinine clearance ≥30 mL/min. Exclusion criteria included previous treatment with an anti-CD38 antibody, such as dara. Pts received intravenous dara at a 16 mg/kg dose weekly for 8 weeks, then every 2 weeks for an additional 16 weeks, followed by 4 weeks interval thereafter. The primary endpoint was the change from baseline in the bone resorption markers C-terminal cross-linking telopeptide of type 1 collagen (CTX) and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b) after 4 months of dara monotherapy. Secondary endpoints included the change from baseline to 4 months of treatment in selected bone formation markers (e.g., bone-specific alkaline phosphatase [bALP], osteocalcin [OC], and procollagen type-I N-propeptide [PINP]), markers of osteoclast regulation (RANKL, osteoprotegerin, dicckopf-1 [DKK-1], sclerostin and C-C motif ligand-3 [CCL3]), progression-free survival (PFS), and overall survival (OS).
Results:
Among the 57 pts enrolled in the study, 33 (57.9%) pts had biomarker and other clinical data available on treatment initiation (baseline) and after 4 months of treatment; the following analyses refer to this pt subgroup. The pts' median (range) age was 73.0 (52.0-84.0) years, and most were female (18, 54.5%). Most (16, 48.5%) pts had >10 lytic bone lesions at baseline. Six (18.2%) pts received bisphosphonates along with dara monotherapy.
The ORR (Partial Response or better [≥PR]) was 63.6% (CR: 3.0%, VGPR: 21.2%, PR: 39.4%). The respective 4-month ORR was 57.6% (VGPR: 24.2%, PR: 33.3%)
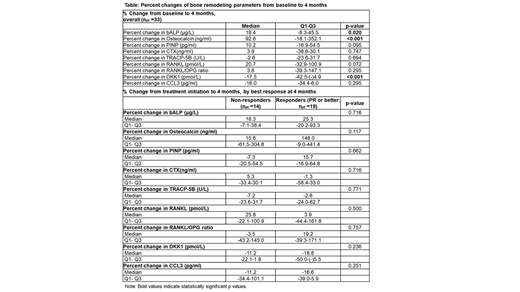
The median CTX change from baseline to 4 months was 3.9%, with 13 (39.4%) and 11 (33.3%) pts exhibiting ≥20% and ≥30% reduction in CTX levels, respectively. The TRACP-5b levels decreased from baseline to 4 months by a median of 2.6%, with 10 (30.3%) and 6 (18.2%) pts showing ≥20% and ≥30% reduction in TRACP-5b levels, respectively. The median changes from baseline to 4 months in the CTX and TRACP-5b levels for pts with a ≥PR at 4 months were -1.3% and -2.6%, respectively; the respective changes for pts not achieving favorable response (i.e., pts with minimal response, stable disease, disease progression, or no response assessment) were 5.3% and -7.2%. The levels of the bone metabolism biomarkers bALP, OC, and PINP increased from baseline to 4 months, the median changes being 18.4%, 92.6% and 10.2%, respectively. For pts with ≥PR at 4 months, the median changes from baseline to 4 months in bALP, OC, and PINP levels were 25.3%, 146.0% and 15.7%, respectively; the respective changes for pts not achieving favourable response were 18.3%, 15.6% and -7.3% (Table 1).
Other significant differences at 4 months included the decrease in DKK-1 by a median of 17.5%, the decrease in CCL3 by 16.0% (Table 1).
The median (95% confidence interval) PFS and OS were 9.3 (6.7-15.3) and 21.2 (11.4-not reached) months, respectively; the respective results for all 57 patients were 4.7 (3.0-7.2) and 10.5 (8.4-18.1) months.
Conclusions:
In these highly pre-treated MM pt group, dara monotherapy showed a positive effect on bone metabolism which was more profound among responders; OC had a ~3-fold increase after 4 months of therapy. The reduction in TRACP-5b and in CCL-3 but not in CTX suggests a mild inhibitory effect on osteoclasts by dara, with a statistically non-significant trend to be greater in responders.
Terpos: Novartis: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Genesis: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Kastritis: Janssen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Genesis Pharma: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Hatjiharissi: Gilead: Honoraria; Genesis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Katodritou: GSK, Amgen, Karyopharm, Abbvie, Janssen-Cilag, Genesis Pharma, Sanofi: Honoraria, Research Funding. Verrou: Amgen: Honoraria; Genesis: Honoraria; Abbvie: Honoraria, Research Funding; Takeda: Honoraria; Roche: Honoraria; Janssen Cilag: Honoraria, Research Funding; Karyopharm: Research Funding. Gavriatopoulou: GSK: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Genesis: Honoraria; Sanofi: Honoraria. Leonidakis: Health Data Specialists: Current Employment. Delimpasi: Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Kyrtsonis: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/Genesis Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria; Sanofi: Membership on an entity's Board of Directors or advisory committees. Symeonidis: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi/Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; MSD: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Demo: Research Funding; Astellas: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; WinMedica: Research Funding; GenesisPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dimopoulos: Beigene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria; Takeda: Honoraria.